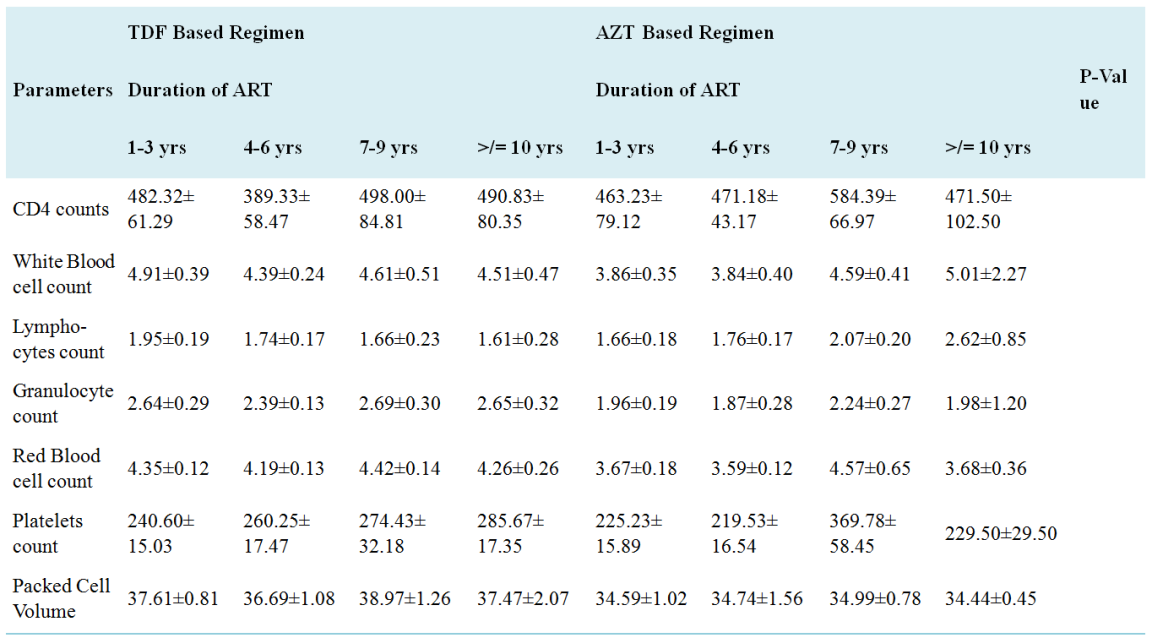
HIV is a global public health concern and people diagnosed with HIV are treated with Antiretroviral therapy. Until 2017, Tenofovir and Zidovudine-based ART were the two major first line drugs for PLHIVs in Nasarawa Nigeria. This study aims to compare the HIV viral load suppression amongst patients on these two ART combinations in Nasarawa State, Nigeria. The study was conducted in three (3) secondary health facilities in Nasarawa State using one hundred subjects selected randomly from the three facilities comprising 50 HIV Sero-positive individuals on Tenofovir-based ART and 50 HIV sero-positive individuals on Zidovudine-based ART. Ethylene diamine Tetra Acetic (EDTA) blood specimen was obtained from each study participant for Full blood count (FBC) using haematology auto-analyser (Sysmex K21N), CD4 count using Partec Cyflow Counter II and HIV viral load analysis using real-time polymerase chain reaction. The demographic data of study participants shows that more females (72) were involved in the study making up 64% of the subjects on Tenofovir and 80% of those on Zidovuine and most of the subjects were within the ages of 26-35years. There was no significant difference (p=0.666) in the viral load of the subjects on any of the regimen. The red blood cells count (RBC) and platelet counts were significantly different (p<0.0001) amongst the subjects on the two ART regimen whereas CD4 count, white blood cells count, lymphocytes count, granulocytes count and Packed cell volume (PCV) were not significantly different within the two groups. Age affected some of the haematological parameters (granulocytes, PCV, RBCs and platelets) within the two groups at different ages. Sex only affected the PCV and granulocytes of subjects within the two different groups (p=0.0069), occupation, knowledge about HIV/AIDS disease and care, duration of ART treatment and year of initial diagnosis of HIV did not affect the haematological and immunological parameters of subjects on the two ART regimen. Conclusively, there is no significant difference in the virologic and immunological response of patients on the two ART therapy but some haematological parameters of subjects on Zidovudine were statistically different from those on Tenofovir.
| Published in | International Journal of HIV/AIDS Prevention, Education and Behavioural Science (Volume 10, Issue 1) |
| DOI | 10.11648/j.ijhpebs.20241001.11 |
| Page(s) | 1-17 |
| Creative Commons |
This is an Open Access article, distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium or format, provided the original work is properly cited. |
| Copyright |
Copyright © The Author(s), 2024. Published by Science Publishing Group |
Tenofovir, Zidovudine, Hematological Parameters, PLHIV, CD4, Viral Load
1.1. Justification for Study
1.2. Research Questions
1.3. Objectives
2.1. Goals of Antiretroviral Therapy
2.1.1. Classes of ARVs and Their Mechanisms of Action
2.1.2. Antiretroviral Drugs-Induced Oxidative Stress as Cause of Cellular Abnormality
2.1.3. CD4-T Cells and HIV
2.1.4. Immunological Staging of HIV Infection
Not significant immunosuppression | >500/mm3 |
Mild immunosuppression | 350 – 499/mm3 |
Advanced immunosuppression | 200 – 349/mm3 |
Severe immunosuppression | <200/mm3 |
2.2. Effect of Haart on CD4 Cell Count
2.3. Effect of Haart on Platelet Count (Thrombocytopaenia)
2.4. Effect of Haart on Platelet Factor-3 Availability
3.1. Study Design
3.2. Sample Size Determination
3.3. Study Population and Recruitment
3.4. Ethical Consideration
3.5. Inclusion Criteria
3.6. Exclusion Criteria
3.7. Data Collection
3.8. Specimen Collection
3.9. Laboratory Analysis
3.10. Data Presetation and Analysis
N (%) | ||
|---|---|---|
Age of participants | ≤15years | 13 (13) |
16-25years | 4 (4) | |
26-35years | 30 (30) | |
36-45years | 28 (28) | |
46-55years | 15 (15) | |
56-65years | 8 (8) | |
>65years | 2 (2) | |
Sex of participants | Female | 72 (72) |
Male | 28 (28) | |
Duration of ART commencement by participants | 1-3YEARS | 38 (38) |
4-6YEARS | 29 (29) | |
7-9YEARS | 25 (25) | |
10YEARS AND ABOVE | 8 (8) | |
Duration of HIV diagnosis of participants | 1-3YEARS | 33 (33) |
4-6YEARS | 35 (35) | |
7-9YEARS | 31 (31) | |
10YEARS AND ABOVE | 11 (11) | |
Occupation of participants | Business | 31 (31) |
Child | 2 (2) | |
Civil Servant | 0 (0) | |
Farmer | 22 (22) | |
Housewife | 12 (12) | |
Student | 19 (19) | |
Subjects on Tenofovir (TDF) Based Regimen | Subjects on Ziduvudine (Azt) Based Regimen | ||
|---|---|---|---|
N (%) | N (%) | ||
Age of participants | ≤15years | 0 (0) | 13 (26) |
16-25years | 4 (8) | 0 (0) | |
26-35years | 17 (34) | 13 (26) | |
36-45years | 16 (32) | 12 (24) | |
46-55years | 8 (16) | 7 (14) | |
56-65years | 3 (6) | 5 (10) | |
>65years | 2 (4) | 0 (0) | |
Sex of participants | Female | 32 (64) | 40 (80) |
Male | 18 (36) | 10 (20) | |
Duration of ART commencement by participants | 1-3YEARS | 25 (50) | 13 (26) |
4-6YEARS | 12 (24) | 17 (34) | |
7-9YEARS | 7 (14) | 18 (36) | |
10YEARS AND ABOVE | 6 (12) | 2 (4) | |
Duration of HIV diagnosis of participants | 1-3YEARS | 11 (22) | 12 (24) |
4-6YEARS | 18 (36) | 17 (34) | |
7-9YEARS | 12 (24) | 19 (38) | |
10YEARS AND ABOVE | 9 (18) | 2 (4) | |
Occupation of participants | Business | 12 (24) | 19 (38) |
Child | 0 (0) | 2 (4) | |
Civil Servant | 0 (0) | 0 (0) | |
Farmer | 15 (30) | 7 (14) | |
Housewife | 9 (18) | 3 (6) | |
Student | 6 (12) | 13 (26) |
Viral Load of participants | Subjects on Tenofovir (TDF) Based Regimen | Subjects on Ziduvudine (AZT) Based Regimen |
|---|---|---|
N (%) | N (%) | |
<20 | 33 (66.0) | 32 (64.0) |
20-50 | 10 (20.0) | 8 (16.0) |
51-200 | 3 (6.0) | 4 (8.0) |
201-1000 | 1 (2.0) | 0 (0.0) |
Above 1000 | 3 (6.0) | 6 (12.0) |
Parameters | Subjects on Tenofovir (TDF) Based Regimen | Subjects on Ziduvudine (AZT) Based Regimen | P-Value |
|---|---|---|---|
CD4 counts | 463.22±36.64 | 509.88±35.19 | 0.3893 |
White Blood cell count | 4.70±0.22 | 4.16±0.23 | 0.3785 |
Lymphocytes count | 1.82±0.11 | 1.88±0.11 | 0.500 |
Granulocyte count | 2.59±0.16 | 2.03±0.15 | 0.3266 |
Red Blood cell count | 4.31±0.08 | 3.97±0.25 | <0.0001 |
Platelets count | 255.46±9.94 | 275.50±24.00 | <0.0001 |
Packed Cell Volume | 37.56±0.56 | 34.78±0.64 | 0.1765 |
Parameters | Age of participants | Subjects on Tenofovir (TDF) Based Regimen | Subjects on Ziduvudine (AZT) Based Regimen | P-Value |
|---|---|---|---|---|
CD4 counts | ≤15years | .- | 538.08±75.60 | NS |
16-25years | 341.25±50.81 | .- | NS | |
26-35years | 591.12±77.03 | 548.62±72.65 | NS | |
36-45years | 351.00±34.30 | 421.67±73.56 | NS | |
46-55years | 476.75±102.93 | 509.86±92.77 | NS | |
56-65years | 399.33±68.05 | 547.60±74.91 | NS | |
>65years | 559.50±290.50 | -. | NS | |
White Blood cell count | ≤15years | .- | 4.95±0.52 | NS |
16-25years | 3.20±0.30 | .- | NS | |
26-35years | 5.55±0.48 | 4.25±0.49 | NS | |
36-45years | 4.13±0.26 | 3.28±0.39 | NS | |
46-55years | 4.83±0.37 | 4.07±0.44 | NS | |
56-65years | 4.42±0.72 | 4.11±0.57 | NS | |
>65years | 4.80±0.51 | -. | NS | |
Lymphocytes count | ≤15years | -. | 2.18±0.22 | NS |
16-25years | 1.40±0.29 | .- | NS | |
26-35years | 2.21±0.23 | 1.98±0.22 | NS | |
36-45years | 1.46±0.12 | 1.59±0.23 | NS | |
46-55years | 1.85±0.27 | 1.75±0.26 | NS | |
56-65years | 1.79±0.61 | 1.74±0.30 | NS | |
>65years | 2.16±0.06 | . | NS | |
Granulocyte count | ≤15years | -. | 2.47±0.34 | NS |
16-25years | 1.63±0.33 | -. | NS | |
26-35years | 2.98±0.38 | 2.04±0.34 | NS | |
36-45years | 2.43±0.20 | 1.48±0.17 | 0.0005 | |
46-55years | 2.70±0.22 | 2.13±0.23 | NS | |
56-65years | 2.34±0.46 | 2.02±0.51 | NS | |
>65years | 2.36±0.48 | .- | NS |
Red Blood cell count | ≤15years | -. | 3.61±0.18 | NS |
16-25years | 4.43±0.31 | -. | NS | |
26-35years | 4.25±0.14 | 3.46±0.10 | <0.0001 | |
36-45years | 4.51±0.12 | 5.32±0.92 | NS | |
46-55years | 4.28±0.16 | 3.74±0.17 | 0.0378 | |
56-65years | 3.74±0.09 | 3.29±0.19 | NS | |
>65years | 3.95±0.26 | .- | NS | |
Platelets count | ≤15years | .- | 271.69±33.59 | NS |
16-25years | 253.75±16.86 | -. | NS | |
26-35years | 248.82±19.22 | 246.92±14.15 | NS | |
36-45years | 237.37±14.25 | 381.75±86.03 | NS | |
46-55years | 255.75±24.08 | 218.43±13.13 | NS | |
56-65years | 314.67±28.29 | 184.60±21.63 | 0.0105 | |
>65years | 370.00±619.00 | .- | NS | |
Packed Cell Volume | ≤15years | .- | 33.73±1.22 | NS |
16-25years | 37.63±1.99 | -. | NS | |
26-35years | 36.21±0.69 | 36.00±0.85 | NS | |
36-45years | 40.20±0.90 | 33.53±1.97 | 0.0025 | |
46-55years | 36.38±1.88 | 36.78±1.01 | NS | |
56-65years | 34.85±1.46 | 34.49±1.32 | NS | |
>65years | 36.63±0.34 | .- | NS |
Subjects on Tenofovir (TDF) Based Regimen | Subjects on Ziduvudine (AZT) Based Regimen | ||||
|---|---|---|---|---|---|
Female | Male | Female | Male | P-Value | |
CD4 counts | 523.47±51.26 | 356.11±34.27 | 520.22±41.67 | 468.50±58.04 | 0.1011 |
White Blood cell count | 4.93±0.30 | 4.27±0.28 | 4.08±0.27 | 4.49±0.46 | 0.1635 |
Lymphocytes count | 1.90±0.15 | 1.67±0.16 | 1.86±0.13 | 1.95±0.21 | 0.4056 |
Granulocyte count | 2.72±0.22 | 2.36±0.19 | 1.97±0.17 | 2.28±0.29 | 0.0399 |
Red Blood cell count | 4.20±0.09 | 4.51±0.12 | 4.02±0.31 | 3.76±0.15 | 0.4466 |
Platelets count | 262.53±11.54 | 242.89±18.59 | 287.13±29.34 | 229.00±21.35 | 0.4863 |
Packed Cell Volume | 36.02±0.49 | 40.29±1.01 | 34.15±0.74 | 37.27±0.92 | 0.0069 |


| [1] | Adesanya, O. & Otulana, Olatoye & Oluwaseun, Huthman & Selimot, Huthman & Ayoola, Adesanya. (2012). Effects of a Single Pill 3-drug Combination of Lamivudine, Nevirapine and Zidovudine on Blood Parameters and Liver Histology in Female Wistar Rats. American Journal of Medicine and Medical Sciences. 2. 71-74. |
| [2] | Agarwal D, Chakravarty J, Chaube L, Rai M, Agrawal NR, Sundar S. High incidence of zidovudine induced anaemia in HIV infected patients in eastern India. Indian J Med Res. 2010; 132: 386–9. [PubMed] [Google Scholar]. |
| [3] |
Akay C, Cooper M, Odeleye A, Jensen BK, White MG, Vassoler F, Gannon PJ, Mankowski J, Dorsey JL, Buch AM, Cross SA, Cook DR, Peña MM, Andersen ES, Christofidou-Solomidou M, Lindl KA, Zink MC, Clements J, Pierce RC, Kolson DL, Jordan-Sciutto KL. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol. 2014 Feb; 20(1): 39-53.
https://doi.org/10.1007/s13365-013-0227-1 . Epub 2014 Jan 14. PMID: 24420448; PMCID: PMC3928514. |
| [4] | Amatore, C., Arbault, S., Jaouen, G., Koh, A. C.., Leong, W. K., Top, S., Valleron, M.-A. and Woo, C. H. (2010), Pro-oxidant Properties of AZT and other Thymidine Analogues in Macrophages: Implication of the Azido Moiety in Oxidative Stress. ChemMedChem, 5: 296-301. |
| [5] | Athe M. N. Tsibris, Martin S. Hirsch, Antiretroviral Therapy for Human Immunodeficiency Virus Infection, Editor(s): John E. Bennett, Raphael Dolin, Martin J. Blaser, Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (Eighth Edition), W. B. Saunders, 2015, Pages 1622-1641. e6, ISBN 9781455748013, |
| [6] | Awofala, A. A. and Ogundele, O. E. (2016) HIV Epidemiology in Nigeria. Saudi Journal of Biological Sciences, 30, 30. |
| [7] | Bai RJ, Dai LL, Wu H. (2020) Advances and challenges in antiretroviral therapy for acquired immunodeficiency syndrome. Chinese medical journal, 133 (23), 2775-2777. |
| [8] | Battistini Garcia SA, Guzman N. Acquired Immune Deficiency Syndrome CD4+ Count. [Updated 2023 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: |
| [9] | Benbrik, E., Chariot, P. and Bonavaud S. (1997). “Cellular and mitochondrial toxicity of zidovudine (AZT), didanosine (ddI) and zalcitabine (ddC) on cultured humanmuscle cells,” Journal of Neurological Science. 149(1): 19–25. |
| [10] |
Bertrand L, Dygert L, Toborek M. Antiretroviral Treatment with Efavirenz Disrupts the Blood-Brain Barrier Integrity and Increases Stroke Severity. Sci Rep. 2016 Dec 23; 6: 39738.
https://doi.org/10.1038/srep39738 . PMID: 28008980; PMCID: PMC5180178. |
| [11] |
Bhatti AB, Usman M, Kandi V. Current Scenario of HIV/AIDS, Treatment Options, and Major Challenges with Compliance to Antiretroviral Therapy. Cureus. 2016 Mar 1; 8(3): e515.
https://doi.org/10.7759/cureus .515. PMID: 27054050; PMCID: PMC4818110. |
| [12] | Biggs, R., Douglas, A. S. and Macfarlane, R. G. (1953). The formation of thromboplastin in human blood. Journal of Physiology. 119: 9. |
| [13] | Bofil, M., Janossy, G., Lee, C. A, MacDonald-Burns, D., Phillips, A. N. and Sabin, C. (1992). Laboratory control values for CD4 and CD8 T lymphocytes. Implications for HIV-1 diagnosis. Clinical Expository Immunology. 88(2): 243–252. |
| [14] | Bouchard, B. A., Mann, K. G. and Butenas, S. (2010). No evidence for tissue factor on platelets. Blood. 116 (5): 854-855. |
| [15] | Broder, S. (2010). The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Research. 85(1): 1–18. |
| [16] | Brummel S, Jeff Stringer, Ed Mills, Camlin Tierney, Ellen C. Caniglia, Angela Colbers, Benjamin H. Chi, Brookie M. Best, Myriam El Gaaloul, Sharon Hillier, Gonzague Jourdain, Saye H. Khoo, Lynne M. Mofenson, Landon Myer, Sharon Nachman, Lynda Stranix-Chibanda, Polly Clayden, Memory Sachikonye, Shahin Lockman. Clinical and population-based study design considerations to accelerate the investigation of new antiretrovirals during pregnancy. |
| [17] | Carme Borrell, Maica Rodríguez-Sanz, M. Isabel Pasarín, M. Teresa Brugal, Patricia García-de-Olalla, Marc Marí-Dell'Olmo, Joan Caylà, AIDS mortality before and after the introduction of highly active antiretroviral therapy: does it vary with socioeconomic group in a country with a National Health System?, European Journal of Public Health, Volume 16, Issue 6, December 2006, Pages 601–608, |
| [18] | CASCADE Collaboration. (2004). Short-term risk of AIDS according to current CD4 cell count and viral load in antiretroviral drug- na¨ıve individuals and those treated in the monotherapy era. AIDS. 18: 51–58. |
| [19] | Caso, J. A. A., Mingo, C. S. and Tena, J. G. (1999). Effects of highly active antiretroviral therapy on thrombocytopenia in patients with HIV infection. National England Journal of Medicine. 341: 1239–1240. |
| [20] | Castaldi, P. A., Larrieu, M. J. and Caen. J. (1965). Availability of platelet factor 3 and activation of factor XII in thrombasthenia. Nature. 207: 422. |
| [21] | Castaldi, P. A., Rozenberg, M. C. and Stewart, J. H. (1966). The bleeding disorder of uraemia. A qualitative platelet defect. Lancet. 2: 66. |
| [22] | Chen, H., Zhao, H. X., Huang, X. F., Chen, G. W. and Yang, Z. X. (2012). Does high load of oxidants in human semen contribute to male factor infertility. Antioxidant Redox Signalling. 16: 754-759. |
| [23] |
Cheung, P. C., Lai W. P., Shuter J. (2017). Zidovudine- versus Tenofovir-Based Antiretroviral Therapy for the Initial Treatment of HIV Infection in the Ethnic Minority Region of Liangshan Prefecture, Sichuan Province, China: An Observational Cohort Study Journal of the International Association of Providers of AIDS Care 2017, Vol. 16(2) 189–193
https://journals.sagepub.com/doi/pdf/10.1177/2325957416686190 |
| [24] | Cummins, Nathan. (2022). Metabolic Complications of Chronic HIV Infection: A Narrative Review. Pathogens. 11. 197. |
| [25] | Dalakas, M. C., Illa, I., Pezeshkpour, G. H., Laukaitis, J. P., Cohen, B. and Griffin, J. L. (1990). Mitochondrial myopathy caused by long-term zidovudine therapy. New England Journal of Medicine. 322(16): 1098–1105. |
| [26] | Dell'Anna, M. L., Ottaviani, M., Bellei, B., Albanesi, V. and Cossarizza, A. (2010). Membrane lipid defects are responsible for the generation of reactive oxygen species in peripheral blood mononuclear cells from vitiligo patients. Journal of Cell Physiology. 223: 187–193. |
| [27] | Ebonyi, A. O., Oguche, S., Dablets, E., Sumi, B., Yakubu, E. and Sagay A. S. (2014). Effect of HAART on growth parameters and absolute CD4 count among HIV-infected children in a rural community of central Nigeria. Nigerian Journal of Paediatrics. 41 (1): 1-6. |
| [28] | Egger, M., May, M., Chene, G., Phillips, A. N., Ledergerber, B. and Dabis, F. (2002). Prognosis of HIV-1 infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 360: 119–129. |
| [29] | Gesesew, H. A., Tesfamichael, F. A. and Adamu B. T. (2013). Factors Affecting Late Presentation for HIV/AIDS Care in Southwest Ethiopia: A Case Control Study. Public Health Research. 3(4): 98–107. |
| [30] | Gortmaker SL, Hughes M, Cervia J, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med 2001; 345: 1522-1528. [PubMed] |
| [31] | Gouripur. T., Desai, P. B., Axita, V., Kranti, G. and Vijayeta, P. (2012). Comparison of Lipid Peroxidation Product And Enzymatic Antioxidants In Newly Diagnosed Pulmonary Tuberculosis Patients With And Without Human Deficiency Virus Infection. International Journal of Pharmaceutical Biological Science. 3(3): 391–397. |
| [32] | Gruszecki, A. C., Wehrli, G. and Ragland, B. D. (2002). Management of a patient with HIV infection-induced anaemia and thrombocytopenia who presented withthrombotic thrombocytopenic purpura. American Journal of Hematology. 69: 22-231. |
| [33] | Hazra Rohan, Siberry K. G. and Mofenson M. L. 2010. Growing Up with HIV: Children, Adolescents, and Young Adults with Perinatally Acquired HIV Infection* Annual Review of Medicine Vol. 61: 169-185 (Volume publication date 18 February 2010) |
| [34] | Hardisty, R. M., and Hutton. R. A (1965). Platelet aggregation and the availability of platelet factor 3. British Journal of Haematology. 12: 764. |
| [35] | Hekimi, S and Guarente, L. (2003). Genetics and the specificity of the aging process. Science. 299: 1351-1354. |
| [36] | Hughes, W., J. A. McDowell, J. Shenep, P. Flynn, M. W. Kline, R. Yogev, W. Symonds, Y. Lou, and S. Hetherington. 1999. Safety and single-dose pharmacokinetics of abacavir (1592U89) in human immunodeficiency virus type 1-infected children. Antimicrob. Agents Chemother. 43: 609-615. [PMC free article] [PubMed] [Google Scholar]. |
| [37] | Jaykaran, C. and Tamoghna, B. (2013). How to calculate sample size for different study designs in medical research? Indian Journal of Pschological Medicine. 35(2). |
| [38] | Johnson, S. A. and Schneider, C. L. (1953). The existence of antifibrinolysin activity in platelets. Science. 117: 229. |
| [39] | Koevary, S. B. (2012). Selective toxicity of rose bengal to ovarian cancer cells in vitro. International Journal of Physiology and Pathophysiological Pharmacology. 4: 99-107. |
| [40] | Leonard Jayne (2023). HIV timelines; What are the stages? |
| [41] |
Lin H, Stankov MV, Hegermann J, Budida R, Panayotova-Dimitrova D, Schmidt RE, Behrens GMN. Zidovudine-Mediated Autophagy Inhibition Enhances Mitochondrial Toxicity in Muscle Cells. Antimicrob Agents Chemother. 2018 Dec 21; 63(1): e01443-18.
https://doi.org/10.1128/AAC.01443-18 . PMID: 30373793; PMCID: PMC6325205. |
| [42] | Meidani, M., Rezaei, F., Maracy, M. R., Avijgan, M., Tayeri, K., 2012. Prevalence, severity, and related factors of anemia in HIV/AIDS patients. Journal of Research in Medical Sciences, 17(2): 138–142. |
| [43] | Mojumdar, K., Vajpayee, M., Chauhan, N. K. and Mendiratta, S. (2010). Late presenters to HIV care and treatment, identification of associated risk factors in HIV-1 infected Indian population. British Medical Journal of Public Health. 10: 416. |
| [44] | Nachman SA, Stanley K, Yogev R, et al. Nucleoside analogs plus ritonavir in stable antiretroviral therapy-experienced HIV-infected children: a randomized controlled trial. Pediatric AIDS clinical trials group 338 study team. JAMA. 2000; 283(4): 492-498. Available at: |
| [45] |
NASCP. 2016. National Guidelines For Hiv Prevention Treatment And Care
https://www.prepwatch.org/wp-content/uploads/2017/08/nigeria_national_guidelines_2016.pdf |
| [46] | Olajide, O., Agbede, T. O., Ajiboye, O. M., Kolawole, S. A., Babatunde, L. Odeigha, O. (2010). Evaluation of Cd4+ T Cells in HIV Patients Presenting With Malaria at The University Of Ilorin Teaching Hospital Nigeria. Exclite Journal. 9: 58-66. |
| [47] | Periayah MH, Halim AS, Mat Saad AZ. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int J Hematol Oncol Stem Cell Res. 2017 Oct 1; 11(4): 319-327. PMID: 29340130; PMCID: PMC5767294. |
| [48] |
Poorolajal J, Hooshmand E, Mahjub H, Esmailnasab N, Jenabi E. Survival rate of AIDS disease and mortality in HIV-infected patients: a meta-analysis. Public Health. 2016 Oct; 139: 3-12.
https://doi.org/10.1016/j.puhe.2016.05.004 . Epub 2016 Jun 24. PMID: 27349729. |
| [49] | Radix A, Shalev N. When to Initiate Antiretroviral Therapy, With Protocol for Rapid Initiation [Internet]. Baltimore (MD): Johns Hopkins University; 2022 Aug. Available from: |
| [50] |
Robbins RN, Spector AY, Mellins CA, Remien RH. Optimizing ART adherence: update for HIV treatment and prevention. Curr HIV/AIDS Rep. 2014 Dec; 11(4): 423-33.
https://doi.org/10.1007/s11904-014-0229-5 . PMID: 25304006; PMCID: PMC4268351. |
| [51] | Scaradavou, A. (2002). HIV related thrombocytopenia. Blood Review. 16: 73-76. |
| [52] |
Shafer RW, Vuitton DA. Highly active antiretroviral therapy (HAART) for the treatment of infection with human immunodeficiency virus type 1. Biomed Pharmacother. 1999 Mar; 53(2): 73-86.
https://doi.org/10.1016/s0753-3322(99)80063-8 . PMID: 10337461. |
| [53] | Singh, R. P., Sharad, S. and Kapur, S. (2004). Free Radicals and Oxidative Stress in Neurodegenerative Diseases: Relevance of Dietary Antioxidants. Journal of Infectious Medicine. 5: 218-225. |
| [54] | Samuel, A., Olowookere, A. A., Fatiregun, J. O., Akinyemi, A. E., Bamgboye, G. K. and Osagbemi, B. (2008). Prevalence and determinants of non-adherence to highly active anti-retroviral therapy among people living with HIV/AIDS in Ibadan, Nigeria. Journal of Infact Developing countries. 2(5): 369–372. |
| [55] | Tegene Legese Dadi, Adane Teshome Kefale, Teshale Ayele Mega, Muktar Sano Kedir, Habtamu Acho Addo, Tessema Tsehay Biru, "Efficacy and Tolerability of Tenofovir Disoproxil Fumarate Based Regimen as Compared to Zidovudine Based Regimens: A Systematic Review and Meta-Analysis", AIDS Research and Treatment, vol. 2017, Article ID 5792925, 7 pages, 2017. |
| [56] | Velen K, Lewis JJ, Charalambous S, Grant AD, Churchyard GJ, Hoffmann CJ. Comparison of tenofovir, zidovudine, or stavudine as part of first-line antiretroviral therapy in a resource-limited-setting: a cohort study. PLoS ONE 2013: 8: e64459. |
| [57] | Vogiatzi, G., Tousoulis, D. and Stefanadis, C. (2009). The role of oxidative stress in atherosclerosis. Hellenic Journal of Cardiology. 50: 402-409. |
| [58] | Watson Stephanie (2022). Antiretroviral HIV Drugs: Side Effects and Adherence. |
| [59] | Weinberg J. L. and Kovarik C. L., Virtual Mentor. 2010; 12(3): 202-206. |
| [60] | WHO. 2005. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance: African region. HIV/AIDS. 1–49. |
| [61] | WHO. 2023. HIV and AIDS. Key facts. |
| [62] | WHO. 2016, Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2nd ed. |
APA Style
Umeozulu, C., Ibeh, N., Obafemi, I. (2024). Comparison of Viral Load, CD4 and Hematological Parameters Amongst HIV, Patients on Tenofovir and Zidovudine-Based ARV Therapy in Nasarawa State, Nigeria. International Journal of HIV/AIDS Prevention, Education and Behavioural Science, 10(1), 1-17. https://doi.org/10.11648/j.ijhpebs.20241001.11
ACS Style
Umeozulu, C.; Ibeh, N.; Obafemi, I. Comparison of Viral Load, CD4 and Hematological Parameters Amongst HIV, Patients on Tenofovir and Zidovudine-Based ARV Therapy in Nasarawa State, Nigeria. Int. J. HIV/AIDS Prev. Educ. Behav. Sci. 2024, 10(1), 1-17. doi: 10.11648/j.ijhpebs.20241001.11
AMA Style
Umeozulu C, Ibeh N, Obafemi I. Comparison of Viral Load, CD4 and Hematological Parameters Amongst HIV, Patients on Tenofovir and Zidovudine-Based ARV Therapy in Nasarawa State, Nigeria. Int J HIV/AIDS Prev Educ Behav Sci. 2024;10(1):1-17. doi: 10.11648/j.ijhpebs.20241001.11
@article{10.11648/j.ijhpebs.20241001.11,
author = {Chinwe Umeozulu and Nnannah Ibeh and Isaac Obafemi},
title = {Comparison of Viral Load, CD4 and Hematological Parameters Amongst HIV, Patients on Tenofovir and Zidovudine-Based ARV Therapy in Nasarawa State, Nigeria
},
journal = {International Journal of HIV/AIDS Prevention, Education and Behavioural Science},
volume = {10},
number = {1},
pages = {1-17},
doi = {10.11648/j.ijhpebs.20241001.11},
url = {https://doi.org/10.11648/j.ijhpebs.20241001.11},
eprint = {https://article.sciencepublishinggroup.com/pdf/10.11648.j.ijhpebs.20241001.11},
abstract = {HIV is a global public health concern and people diagnosed with HIV are treated with Antiretroviral therapy. Until 2017, Tenofovir and Zidovudine-based ART were the two major first line drugs for PLHIVs in Nasarawa Nigeria. This study aims to compare the HIV viral load suppression amongst patients on these two ART combinations in Nasarawa State, Nigeria. The study was conducted in three (3) secondary health facilities in Nasarawa State using one hundred subjects selected randomly from the three facilities comprising 50 HIV Sero-positive individuals on Tenofovir-based ART and 50 HIV sero-positive individuals on Zidovudine-based ART. Ethylene diamine Tetra Acetic (EDTA) blood specimen was obtained from each study participant for Full blood count (FBC) using haematology auto-analyser (Sysmex K21N), CD4 count using Partec Cyflow Counter II and HIV viral load analysis using real-time polymerase chain reaction. The demographic data of study participants shows that more females (72) were involved in the study making up 64% of the subjects on Tenofovir and 80% of those on Zidovuine and most of the subjects were within the ages of 26-35years. There was no significant difference (p=0.666) in the viral load of the subjects on any of the regimen. The red blood cells count (RBC) and platelet counts were significantly different (p<0.0001) amongst the subjects on the two ART regimen whereas CD4 count, white blood cells count, lymphocytes count, granulocytes count and Packed cell volume (PCV) were not significantly different within the two groups. Age affected some of the haematological parameters (granulocytes, PCV, RBCs and platelets) within the two groups at different ages. Sex only affected the PCV and granulocytes of subjects within the two different groups (p=0.0069), occupation, knowledge about HIV/AIDS disease and care, duration of ART treatment and year of initial diagnosis of HIV did not affect the haematological and immunological parameters of subjects on the two ART regimen. Conclusively, there is no significant difference in the virologic and immunological response of patients on the two ART therapy but some haematological parameters of subjects on Zidovudine were statistically different from those on Tenofovir.
},
year = {2024}
}
TY - JOUR T1 - Comparison of Viral Load, CD4 and Hematological Parameters Amongst HIV, Patients on Tenofovir and Zidovudine-Based ARV Therapy in Nasarawa State, Nigeria AU - Chinwe Umeozulu AU - Nnannah Ibeh AU - Isaac Obafemi Y1 - 2024/04/02 PY - 2024 N1 - https://doi.org/10.11648/j.ijhpebs.20241001.11 DO - 10.11648/j.ijhpebs.20241001.11 T2 - International Journal of HIV/AIDS Prevention, Education and Behavioural Science JF - International Journal of HIV/AIDS Prevention, Education and Behavioural Science JO - International Journal of HIV/AIDS Prevention, Education and Behavioural Science SP - 1 EP - 17 PB - Science Publishing Group SN - 2575-5765 UR - https://doi.org/10.11648/j.ijhpebs.20241001.11 AB - HIV is a global public health concern and people diagnosed with HIV are treated with Antiretroviral therapy. Until 2017, Tenofovir and Zidovudine-based ART were the two major first line drugs for PLHIVs in Nasarawa Nigeria. This study aims to compare the HIV viral load suppression amongst patients on these two ART combinations in Nasarawa State, Nigeria. The study was conducted in three (3) secondary health facilities in Nasarawa State using one hundred subjects selected randomly from the three facilities comprising 50 HIV Sero-positive individuals on Tenofovir-based ART and 50 HIV sero-positive individuals on Zidovudine-based ART. Ethylene diamine Tetra Acetic (EDTA) blood specimen was obtained from each study participant for Full blood count (FBC) using haematology auto-analyser (Sysmex K21N), CD4 count using Partec Cyflow Counter II and HIV viral load analysis using real-time polymerase chain reaction. The demographic data of study participants shows that more females (72) were involved in the study making up 64% of the subjects on Tenofovir and 80% of those on Zidovuine and most of the subjects were within the ages of 26-35years. There was no significant difference (p=0.666) in the viral load of the subjects on any of the regimen. The red blood cells count (RBC) and platelet counts were significantly different (p<0.0001) amongst the subjects on the two ART regimen whereas CD4 count, white blood cells count, lymphocytes count, granulocytes count and Packed cell volume (PCV) were not significantly different within the two groups. Age affected some of the haematological parameters (granulocytes, PCV, RBCs and platelets) within the two groups at different ages. Sex only affected the PCV and granulocytes of subjects within the two different groups (p=0.0069), occupation, knowledge about HIV/AIDS disease and care, duration of ART treatment and year of initial diagnosis of HIV did not affect the haematological and immunological parameters of subjects on the two ART regimen. Conclusively, there is no significant difference in the virologic and immunological response of patients on the two ART therapy but some haematological parameters of subjects on Zidovudine were statistically different from those on Tenofovir. VL - 10 IS - 1 ER -